A Professional Networking Site for Doctors & Medical Students Worldwide
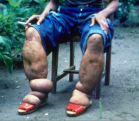
The FDA has said that the controversial drug Avastin should be phased out as a treatment for metastatic breast cancer. Recent studies show that its benefits are outweighed by dangerous side effects.
The announcement does not affect Avastin's status as a drug that can be prescribed for lung cancer, kidney cancer, colorectal cancer and brain cancer.
In 2008, the FDA granted Avastin accelerated approval for use to treat metastatic breast cancer. But studies have failed to show that patients getting Avastin lived longer than patients on standard chemotherapies.
According to CNN:
"Along with those disappointing findings, serious side effects became apparent in patients taking Avastin, including high blood pressure, internal bleeding, perforated internal organs, heart failure and heart attacks, and in some cases, even swelling of the brain."
Genentech, which makes Avastin, has a right to appeal the decision.
Views: 6
© 2013 Doctors Hangout | About DH